Automobile Emissions: An Overview
Emissions from an individual car are
generally low, relative to the smokestack image many people associate
with air pollution. But in numerous cities across the country, the
personal automobile is the single greatest polluter, as
emissions from millions of vehicles on the road add up. Driving a
private car is probably a typical citizen's most "polluting" daily
activity.
Sources of Auto Emissions
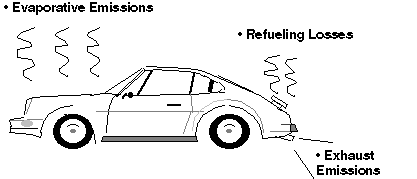
The power to move a car comes from burning
fuel in an engine. Pollution from cars comes from by-products of this
combustion process (exhaust) and from evaporation of the fuel itself.
The
Combustion Process
Gasoline and diesel fuels are mixtures of
hydrocarbons, compounds which contain hydrogen and carbon atoms. In a
"perfect" engine, oxygen in the air would convert all the hydrogen in
the fuel to water and all the carbon in the fuel to carbon dioxide.
Nitrogen in the air would remain unaffected. In reality, the combustion
process cannot be "perfect," and automotive engines emit several types
of pollutants. Pollutants also
escape into the air through fuel evaporation
•
"Perfect" Combustion:
FUEL (hydrocarbons) + AIR (oxygen and
nitrogen) ==>> CARBON DIOXIDE + water + unaffected nitrogen
•
Typical Engine Combustion:
FUEL + AIR ==>> UNBURNED HYDROCARBONS
+ NITROGEN OXIDES + CARBON MONOXIDE + CARBON DIOXIDE + water
-
Back
to Day 1 Lesson- -Go
to Day 3-4 Lesson- -Go
to Day 3-4 Lesson for Davea-
There
are four major pollutants from vehicle emissions:
•
HYDROCARBONS
Hydrocarbon
emissions result when fuel molecules in the engine do not burn or burn
only partially. Hydrocarbons react in the presence of nitrogen oxides
and sunlight to form ground-level ozone, a major component of smog.
Ozone irritates the eyes, damages the lungs, and aggravates respiratory
problems. It is our most widespread and intractable urban air pollution
problem. A number of exhaust hydrocarbons are also toxic, with the
potential to cause cancer.
•
NITROGEN OXIDES (NOx)
Under the high
pressure and temperature conditions in an engine, nitrogen and oxygen
atoms in the air react to form various nitrogen oxides, collectively
known as NOx. Nitrogen oxides, like hydrocarbons, are
precursors to the formation of ozone. They also contribute to the
formation of acid rain.
•
CARBON MONOXIDE
Carbon
monoxide (CO) is a product of incomplete combustion and occurs when
carbon in the fuel is partially oxidized rather than fully oxidized to
carbon dioxide (CO ). Carbon monoxide reduces the flow of oxygen in the
bloodstream and is particularly dangerous to persons with heart
disease.
•
CARBON DIOXIDE
In recent
years, the U.S. Environmental Protection Agency (EPA) has started to
view carbon dioxide, a product of "perfect" combustion, as a
pollution concern. Carbon dioxide does not directly impair human
health, but it is a "greenhouse gas" that traps the earth's heat and
contributes to the potential for global warming.
Fuel
Evaporation
Hydrocarbon pollutants also escape into the
air through fuel evaporation. With today's efficient exhaust emission
controls and today's gasoline formulations, evaporative losses can
account for a majority of the total hydrocarbon pollution from current
model cars on hot days when ozone levels are highest. Evaporative
emissions occur several ways:
•
DIURNAL: Gasoline evaporation
increases as the temperature rises during the day, heating the fuel
tank and venting gasoline vapors.
•
RUNNING LOSSES: The hot
engine and exhaust system can vaporize gasoline when the car is
running.
•
HOT SOAK: The engine
remains
hot for a period of time after the car is turned off, and gasoline
evaporation continues when the car is parked. •REFUELING: Gasoline vapors are always present in
fuel tanks. These vapors are forced out when the tank is filled with
liquid fuel.



