Suggested Experiments
- Allow students to qualitatively see the difference between the ionic and molecular nature of substance in aqueous solution. This can include differences in strength of weak acids and bases, or the number of ions that an ionic substance dissociates into per formula unit.
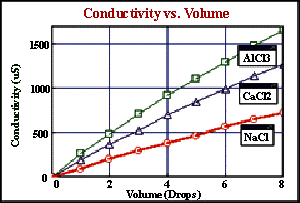
- Use the probe to confirm the direct relationship between conductivity and ion concentration in an aqueous solution. Concentrations of unknown samples can then be determined.
- Measure changes in conductivity resulting from photosynthesis in aquatic plants, with the resulting decrease in bicarbonate-ion concentration from carbon dioxide.
- Use the sensor for an accurate on-site measurement of total dissolved solids in a stream or lake survey.
- Monitor the rate of reaction in a chemical reaction in which dissolved ions and solution conductivity varies with time due to an ionic specie being consumed or produced.
- Perform a conductivity titration to determine when stoichiometric quantities of two substances have been combined.
- Use the probe to determine the rate at which an ionic species diffuses through a membrane, such as dialysis tubing.
- Monitor changes in conductivity or total dissolved solids in an aquarium containing aquatic plants and animals. These changes could be due to photosynthesis or respiration.
 Measuring Conductivity of NaCl
Measuring Conductivity of NaCl